Cancer stem cells have been identified in acute myeloid leukemia and in breast, lung, colon, pancreatic, brain and prostate tumors. There is increasing evidence that standard treatments like chemo just kill the dividing “daughter” (progenitor) cells, without killing the cancer stem cell that neither divides rapidly nor dies. This is especially relevant for young women, as they have “aggressive” cancers, and although current therapies can stop the rapidly dividing daughter progenitor cancer cells. Women still relapse because the chemo has not affected or killed the therapy-resistant cancer stem cells that have the ability, when activated, to give rise to many daughter cells of high proliferate potential. It’s like trying to weed the garden. It’s no good just chopping off the leaves, we need to target the roots to stop the weeds coming back. The problem is that cancer stem cells are rare and difficult to study in the lab because they quickly change into other types of cells. And they are hard to kill.
Are there ways to valiantly fight many types of cancer stem cells? You bet there is.
It has been suggested that combined effect of natural agents may improve the treatment effectiveness in combating proliferation of cancer cells. Plants have been utilized as medicines for thousands of years. The use of medicinal plant was then developed into anticancer drugs. More recently, drug discovery techniques have been moved on to the use of combined active agents where they are believed to be more active as compared to the single agent itself.
To date, other than the fact that many targets are present on the cancer stem cells, the outcome of an agent targeting a single molecule has been disappointing. The simplest reason could be that cancer stem cells have many molecular targets, which function aberrantly, and therefore, for the best results in treating cancer, a multi-molecular targeting approach is essential. In this regard, natural agents, such as Genistein, Parthenolide, Sulforaphane, Curcumin and Chemorine, usually have many targets for their effective action, and therefore, these agents could be of potential use in treating breast cancer. These could be used to target and kill other cancer/leukemia stem cells as well. Genistein, Parthenolide, Sulforaphane, Curcumin and Chemorine are extremely synergistic in the killing of cancer stem cells.
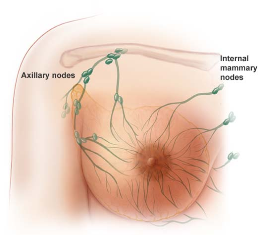 Mammary stem cells are located within a specialized niche in the basal epithelial compartment that is under strict local and systemic regulation. A new study shows that the ovarian hormone progesterone plays a pivotal role in altering breast stem cells. This study shows how and when hormones affect breast stem cells during the natural reproductive cycle. There are well accepted links between ovarian hormones and breast cancer, and there is mounting evidence that stem cells are seeds for breast cancer. We now know a direct connection between progesterone hormones and breast stem cells.
Mammary stem cells are located within a specialized niche in the basal epithelial compartment that is under strict local and systemic regulation. A new study shows that the ovarian hormone progesterone plays a pivotal role in altering breast stem cells. This study shows how and when hormones affect breast stem cells during the natural reproductive cycle. There are well accepted links between ovarian hormones and breast cancer, and there is mounting evidence that stem cells are seeds for breast cancer. We now know a direct connection between progesterone hormones and breast stem cells.
Progesterone induces adult mammary stem cell expansion.
Targeting breast cancer stem cells.
Cancer stem cells and the cell cycle: targeting the drive behind breast cancer.
Well, genistein is back.
Study showed that genistein sufficiently reduced progesterone production in trophoblast cells. In addition, a stimulating effect on estrogen production by genistein was observed. Stimulation of estrogen production by genistein in trophoblast cells is probably due to estrogen receptor blocking effects of genistein. Trophoblast cells seem to compensate blocking of its estrogen receptors by higher estrogen production.
Notch signaling plays a critical role in maintaining the balance between cell proliferation, differentiation, and apoptosis. Researchers have uncovered important roles for Notch genes in regulating breast development and function. This discovery has important implications for breast cancer, since elevated levels of Notch have been linked to breast cancer. The Notch pathway sends signals from a cell’s surface membrane into its nucleus. Those signals activate genes that instruct the cell to make proteins that perform various tasks.
First, Notch helps restrict breast stem cell number, so that when Notch is ’switched off’, there is a resultant expansion in breast stem cells.
Second, Notch is important for ensuring that stem cells produce the sleeve of cells that normally line breast ducts. These ‘luminal’ cells may be the cells that give rise to common types of breast cancer.
Thus, Notch helps to orchestrate the formation of breast tissue: it plays an important role in controlling stem cell number and instructs stem cells to produce luminal cells.
Notch pathway as candidate therapeutic target in Her2/Neu/ErbB2 receptor-negative breast tumors.
Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor.
HER-2, notch, and breast cancer stem cells: targeting an axis of evil.
Genistein inhibits cell growth and induces apoptotic processes mediated via Notch signaling pathway. Curcumin is associated with the inhibition of cell growth mediated via Notch signaling too.
Notch1 augments NF-kappaB activity by facilitating its nuclear retention.
Cancer stem cells are plastic cell states governed by microenvironmental conditions, such as hypoxia, that may be critical for the development of new therapies targeted to disrupt the microenvironment. We are all aware that aerobic glycolysis fuels cancer cell growth. If you block glycolysis in cancer cells, they immediately die. Glycolysis is maintained in cells by two different compounds. HIF-1, hypoxia induction factor, stimulates the synthesis of the enzymes involved in glycolysis. Genistein inhibits HIF-1.
The enzyme AKT phosphorylates and activates these enzymes. AKT stimulates aerobic glycolysis in cancer cells. Genistein inhibits AKT activity by two different methods. First, it apparently directly interferes with AKT phosphorylation, and thereby activation. This reduces glucose consumption in cancer cells and promotes death by both apoptosis and autophagocytosis. Second, genistein activates the PTEN gene. This tumor suppressor inhibits the PI-3K/Akt signaling pathway by blocking the ability of the enzyme PI-3K to activate AKT. In addition to inhibiting glucose metabolism via glycolysis, AKT is a major survival factor for all cancer and leukemia cells.
Hypoxia inducible factors in cancer stem cells.
Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha.
Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells.
Inhibitory effect of isoflavones on prostate cancer cells and PTEN gene.
Genistein-induced apoptosis via Akt signaling pathway in anaplastic large-cell lymphoma.
Phytoestrogen exposure elevates PTEN levels.
Akt stimulates aerobic glycolysis in cancer cells.
Regulation of sensitivity to TRAIL by the PTEN tumor suppressor.
However, remember that genistein is practically insoluble in water and is not suitable for human consumption. Dietary genistein in supplement form didn’t help cancers due to extremely low solubility/bioavailability, which means that most of what we swallow goes directly into our gastrointestinal area and is expelled. In order to introduce pure genistein into the blood via absorption and maximize the activity of genistein in the body, you have to use GenisZym. Otherwise, genistein wouldn’t have worked.