Melatonin, the so-called sleep hormone, is a molecule secreted by the pineal gland – a pea-sized gland located just above the middle of the brain. During the day the pineal is inactive. When the sun goes down and absolute darkness occurs, the pineal is “turned on” and begins to actively produce melatonin, which is released into the blood. Thus, serum melatonin levels have been found to be high at night (80–120pg/ml) and low during the day (2–20pg/ml).
The pattern of waking during the day when it is light and sleeping at night when it is dark is a natural part of human life. Only recently have scientists begun to understand the alternating cycle of sleep and waking, and how it is related to daylight and darkness.
In addition to its well-known regulatory control of the sleep/wake cycle, as well as circadian rhythms generally, melatonin is involved in immunomodulation, hematopoiesis, and antioxidative processes. It is the controlling hormone for many of the diurnal cycles that exist in the body. It has beneficial effects on everything from heart disease and diabetes, to bone health and obesity. And best of all, emerging science now suggests that it may protect our genetic material and guard against age related disease and decline.
Melatonin is a known anti-carcinogen and promotor of cell mediated immunity. The majority of the epidemiological studies performed to date have focused on the association between shift work and breast cancer risk, few studies have reported an increased risk of other cancers, including colorectal cancer, endometrial cancer, prostate cancer and non-Hodgkin’s lymphoma.
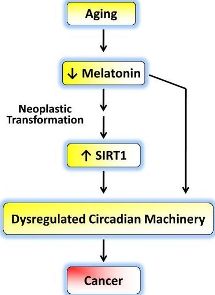 It is now generally well accepted that aging is a major risk factor for prostate cancer. Studies show that men with prostate cancer have lower melatonin levels than men without the disease. The SIRT1 deacetylase may be one of the most important enzymes in the body. Aging is associated with reduced levels of melatonin and various age-related neoplasms have elevated levels of SIRT1. The enhanced SIRT1 levels in several cancers occur at a time when melatonin levels are reduced.
It is now generally well accepted that aging is a major risk factor for prostate cancer. Studies show that men with prostate cancer have lower melatonin levels than men without the disease. The SIRT1 deacetylase may be one of the most important enzymes in the body. Aging is associated with reduced levels of melatonin and various age-related neoplasms have elevated levels of SIRT1. The enhanced SIRT1 levels in several cancers occur at a time when melatonin levels are reduced.
Reduced melatonin levels as well as increased SIRT1 activity in cancer cells may well lead to alterations in the biological clock causing a dysregulation of circadian rhythms thereby leading to uncontrolled cell cycle progression, finally culminating in cancer development. Thus, exogenous melatonin administration may lead to SIRT1 inhibition that could be responsible for melatonin’s anti-cancer effects. Clearly, the lack of melatonin correlates with a predisposition to develop cancers. Melatonin has the ability to interfere with cancer cell multiplication and growth, as well as inducing cancer cell death (apoptosis).
The mechanisms of action of melatonin include the involvement of membrane receptors (MT1, MT2), cytosolic binding sites (MT3 and calmodulin), and nuclear receptors of the RZR/ROR family. Melatonin’s anti-cancer propertries involve activation of both membrane and nuclear receptors, and the inhibition of calmodulin activity. Melatonin also has receptor-independent activity and can directly scavenge free radicals.
Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer.
SIRT1 is significantly elevated in mouse and human prostate cancer.
Physiology and pharmacology of melatonin in relation to biological rhythms.
Melatonin, immune function and cancer.
Molecular mechanisms of melatonin anticancer effects.
Therapeutic actions of melatonin in cancer: possible mechanisms.
There is no evidence that androgens cause sustained cell proliferation in the prostate cancer. It is likely that there are various genetic and non-genetic, hormonal and non-hormonal factors. Probably these factors interact in highly complex and poorly understood ways. Testosterone can be converted to estrogen (17β-estradiol) by the enzyme aromatase, which is expressed in the fat tissue and in prostates. 17β-estradiol up-regulates the IGF-R (insulin-like growth factor receptor), which could faciliate the development of bone metastases because IGFs are required for cell growth.
The role for estrogens in the prostate and in prostate disease is complex and is still only just beginning to be appreciated. Estrogens are critical players in human prostate cancer; their role has been only recently reconsidered. Prostate contains estrogen receptor alpha (ER-alpha) and beta (ER-beta). We are also beginning to recognize the importance and differential roles of the estrogen receptors.
Specifically, the activation of ER-alpha leads to aberrant proliferation, inflammation, and the development of premalignant lesions, while, in contrast, the activation of ER-beta is critical in prostatic stromal-epithelial cell signaling and mediating antiproliferative effects that balance the proliferative action of androgens on the epithelia. Estrogens have a critical role, therefore, in prostate tumor progression to androgen independence.
Melatonin is a natural inhibitor of estrogen binding to its receptor. Further, melatonin inhibits aromatase activity and expression, the enzyme that converts testosterone to estrogen in peripheral tissues, by regulating the gene expression of specific aromatase promoter regions.
The dual, opposing roles of estrogen in the prostate.
Essential role for estrogen receptor beta in stromal-epithelial regulation of prostatic hyperplasia.
Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer.
Hormones and prostate carcinogenesis: Androgens and estrogens.
Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario.
Melatonin as a selective estrogen enzyme modulator.
Melatonin suppresses aromatase expression and activity in breast cancer associated fibroblasts.
Breast cancer therapy based on melatonin.
Melatonin has antiproliferative effects in androgen depentdent and independent prostate cancer cells. The concentration of melatonin necessary to inhibit cell growth is much higher than its blood physiological concentrations in prostate tumor. In one small-scale study, melatonin – combined with conventional medical treatment – improved survival rates in 9 out of 14 men with metastatic prostate cancer.
There is currently no recommended dose for melatonin supplements. Different people will have different responses to its effects. However, therapeutic implications of melatonin should be started at 3-6 mg per day (1 hour before bedtime is usually effective) and then gradually increased to a therapeutic dosage. Generally, the total dosage should not exceed 10 mg per day. Higher doses may cause anxiety and irritability. Interestingly, since meditation may cause melatonin levels to rise it appears to be a valuable addition to the treatment of prostate cancer.
Monitoring intracellular melatonin levels in human prostate normal and cancer cells by HPLC.
Growth-inhibitory activity of melatonin on human androgen-independent DU 145 prostate cancer cells.
Antiproliferative action of melatonin on human prostate cancer LNCaP cells.
Detection of nighttime melatonin level in Chinese Original Quiet Sitting.