Apoptosis is a complex process, where important role is played by epigenetic mechanisms responsible for regulation of activity of proteins participating in this event. The presence of various forms of a given protein (as a result of alternative splicing) and post translation modification of protein molecules (phosphorylation, dephosphorylation, proteolytic cleavage) means that each protein can function as both proapoptotic and antiapoptotic factor. There are two major ways that could downregulate cancer cell apoptosis: (1) somatic and nonsomatic mutation and loss of expression of proapoptotic molecules; and (2) overexpression of apoptosis inhibitory molecules.
Some viruses associated with cancers use tricks to prevent apoptosis of the cells they have transformed. Several human papilloma viruses (HPV) have been implicated in causing cervical cancer. One of them produces a protein (E6) that binds and inactivates the apoptosis promoter p53. Epstein barr virus (EBV), the cause of mononucleosis and associated with some lymphomas produces a protein similar to Bcl-2. EBV produces another protein that causes the cell to increase its own production of Bcl-2. Both these actions make the cell more resistant to apoptosis (thus enabling a cancer cell to continue to proliferate).
Even cancer cells produced without the participation of viruses may have tricks to avoid apoptosis. Some B-cell leukemias and lymphomas express high levels of Bcl-2, thus blocking apoptotic signals they may receive. The high levels result from a translocation of the BCL-2 gene into an enhancer region for antibody production. Melanoma (the most dangerous type of skin cancer) cells avoid apoptosis by inhibiting the expression of the gene encoding Apaf-1.
Some cancer cells, especially lung and colon cancer cells secrete elevated levels of a soluble “decoy” molecule that binds to FasL, plugging it up so it cannot bind Fas. Thus, cytotoxic T cells (CTL) cannot kill the cancer cells by the mechanism shown above. Other cancer cells express high levels of FasL, and can kill any cytotoxic T cells (CTL) that try to kill them because CTL also express Fas (but are protected from their own FasL).
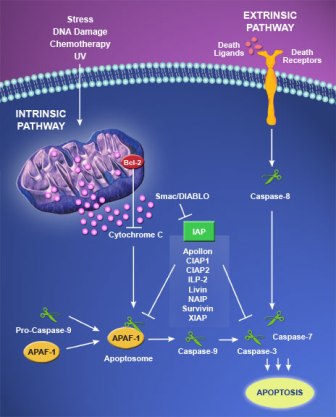 Caspases are the main “executioners” of apoptosis that initiate and propagate the apoptosis, and finally degrade target molecules. In mammals, there are 14 caspases, of which many participate in the apoptotic pathways. Caspase-encoding genes have been reported to harbor inactivating mutations in many human cancers. The IAP (inhibitor of apoptosis) proteins inhibit apoptosis by binding to activated caspases. They inhibit signals generated through both the two major pathways of apoptosis: the Extrinsic (death receptor mediated) and the Intrinsic (mitochondrial mediated) pathways. IAP proteins were originally discovered in baculovirus as suppressors of host cell apoptosis; however, they can be found in both invertebrates and vertebrates. Thus far, eight human IAPs family members have been identified including neuronal apoptosis inhibitory protein (NAIP) (also known as BIRC1), cellular IAP 1 (also known as BIRC2), cIAP2 (also known as BIRC3), X-chromosome linked IAP or XIAP (also known as BIRC4), survivin (also known as BIRC5), Apollon (also known as BIRC6), melanoma IAP (also known as livin, and BIRC7), and IAPlike protein 2 (also known as BIRC8), which are reviewed elsewhere.
Caspases are the main “executioners” of apoptosis that initiate and propagate the apoptosis, and finally degrade target molecules. In mammals, there are 14 caspases, of which many participate in the apoptotic pathways. Caspase-encoding genes have been reported to harbor inactivating mutations in many human cancers. The IAP (inhibitor of apoptosis) proteins inhibit apoptosis by binding to activated caspases. They inhibit signals generated through both the two major pathways of apoptosis: the Extrinsic (death receptor mediated) and the Intrinsic (mitochondrial mediated) pathways. IAP proteins were originally discovered in baculovirus as suppressors of host cell apoptosis; however, they can be found in both invertebrates and vertebrates. Thus far, eight human IAPs family members have been identified including neuronal apoptosis inhibitory protein (NAIP) (also known as BIRC1), cellular IAP 1 (also known as BIRC2), cIAP2 (also known as BIRC3), X-chromosome linked IAP or XIAP (also known as BIRC4), survivin (also known as BIRC5), Apollon (also known as BIRC6), melanoma IAP (also known as livin, and BIRC7), and IAPlike protein 2 (also known as BIRC8), which are reviewed elsewhere.
All IAP proteins share two to three common structures of baculovirus IAP repeat (BIR) domains to bind and inactivate caspases, except survivin, the smallest human IAP protein, which contains only a single BIR repeat. Most of the IAP proteins, excluding survivin, possess a carboxyl-terminal RING domain containing ubiquitin ligases required for ubiquitination and proteasomal degradation of caspases. XIAP is the most efficient caspase inhibitor among the IAP family members.Inhibition of apoptosis by XIAP is mainly coordinated through binding to initiator caspase-9 and effector caspases-3 and -7. Caspase-3 is one of the key executioners of apoptosis, being responsible either partially or totally for the proteolytic cleavage of many key proteins.
Assembling the building blocks: structure and function of inhibitor of apoptosis proteins.
IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer.
Caspases and cancer: mechanisms of inactivation and new treatment modalities.
Targeting apoptosis in prostate cancer: focus on caspases and inhibitors of apoptosis proteins.
Protein kinase Cdelta and caspase-3 modulate TRAIL-induced apoptosis in breast tumor cells.
 Baikal skullcap is a plant native to southern China and all of Korea. It is commonly used in herbalism, where it is considered to be one of the 50 fundamental herbs and is used primarily in treating “hot and damp” conditions such as dysentery and diarrhea. In traditional medicine, it is used as an anti-inflammatory, diuretic, and antitumor agent, especially in liver diseases such as hepatitis and liver cancer. In Western herbalism, it is better known as an ingredient in PC-SPES (PC-SPES has been recalled from the U.S market and should not be used).
Baikal skullcap is a plant native to southern China and all of Korea. It is commonly used in herbalism, where it is considered to be one of the 50 fundamental herbs and is used primarily in treating “hot and damp” conditions such as dysentery and diarrhea. In traditional medicine, it is used as an anti-inflammatory, diuretic, and antitumor agent, especially in liver diseases such as hepatitis and liver cancer. In Western herbalism, it is better known as an ingredient in PC-SPES (PC-SPES has been recalled from the U.S market and should not be used).
Baicalein is a flavonoid originally isolated from the roots of Baikal skullcap (Scutellaria baicalensis Georgi). Several different functions of baicalein have been reported. Study shows that baicalein stimulates apoptosis through the caspase-independent pathway, while undergoing apoptosis, there was a remarkable accumulation of G2/M cells. Also, the ratio of Bax/Bcl-2 was increased leading to changes in mitochondria membrane potential (DeltaPsim) and release of cytochrome c, whereas the baicalein-induced apoptosis was partially abrogated by pretreatment with the pan-caspase inhibitor, the accumulation of G2/M cells remained. These results demonstrate that the cytotoxicity of baicalein is attributable to apoptosis mainly involving G2/M-arrest in an ER-dependent manner, via a mitochondria-dependent caspase pathway and as well as contributions of AIF and Endo G pathways. Moreover, another study shows that Baicalein induced apoptosis through the inhibition of Bcl-2 expression, increased the levels of Bax, reduced the level of deltapsim, and promoted the cytochrome c release and caspase-3 activation. In conclusion, baicalein induced apoptosis via Ca2+ production, mitochondria-dependent and caspase-3 activation in breast cancer cells.
Molecular crosstalk between TRAIL and natural antioxidants in the treatment of cancer.
The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells.
 Eating the whole apple might offer some anti-cancer benefits. Apples contain several classes of polyphenols: monomers (catechins, epicatechins) and oligomers/polymers, such as the procyanidins. The following studies show that the apple procyanidins increased mitochondrial membrane permeability and cytochrome c release from mitochondria and activated caspase-3 and caspase-9 within the tumor cells.
Eating the whole apple might offer some anti-cancer benefits. Apples contain several classes of polyphenols: monomers (catechins, epicatechins) and oligomers/polymers, such as the procyanidins. The following studies show that the apple procyanidins increased mitochondrial membrane permeability and cytochrome c release from mitochondria and activated caspase-3 and caspase-9 within the tumor cells.
Apple procyanidins plus lysosomotropic drugs (phenylalanine methylester and chloroquine) combination amplified procyanidin-induced growth inhibition and apoptosis in cancer cells at non-cytotoxic concentrations. The improved toxicity of the drug combinations relies primarily on the enhancement of lysosomal membrane permeability. Lysosomotropic drugs can enter selectively the lysosomes of certain cell types; may be useful in chemotherapy for destruction of tumor cells. Also, Combinations with quercetin improve the anti-cancer properties of apple procyanidins.
Triggering liposomal drug release with a lysosomotropic agent.
Lysosomotropic drugs inhibit maturation of transforming growth factor-beta.