A number of recent anti-cancer strategies aim at targeting the mitochondrial apoptotic machinery to induce tumor cell death. Apoptosis is a form of cell death in which a programmed sequence of events leads to the elimination of cells. The term apoptosis is derived from the Greek word that signifies the dropping of leaves from the trees. Apoptosis plays a crucial role in developing and maintaining health by eliminating old cells, unnecessary cells, and unhealthy cells. The human body replaces perhaps a million cells a second. Too little or too much apoptosis plays a role in a great many diseases.
There is a distinct and precisely localized control over the fate of specific cells in a mixed cell population that undergo apoptosis. This event similar to proliferation is tightly regulated with both processes playing essential roles in the homeostasis of renewable tissues. When programmed cell death does not work right, cells that should be eliminated may hang around and become immortal. For example, in cancer and leukemia, when apoptosis works overly well, it kills too many cells and inflicts grave tissue damage. This is the case in strokes and neurodegenerative disorders such as Alzheimer, Huntington and Parkinson diseases.
In mammalian cells, apoptosis has been divided into two major pathways: the extrinsic pathway, activated by pro-apoptotic receptor signals at the cellular surface; and the intrinsic pathway, which involves the disruption of mitochondrial membrane integrity. Stress in the endoplasmic reticulum (ER) can also result in apoptosis. Apoptosis is regulated by:
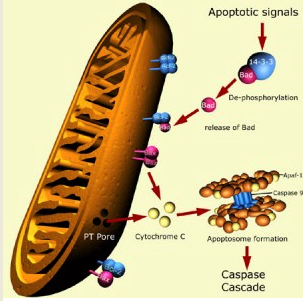 In most cells, these pathways are controlled by the Bcl-2 family of proteins that can be divided into anti-apoptotic and pro-apoptotic members. In the mitochondrial pathway of apoptosis, caspase activation is closely linked to mitochondrial outer membrane permeabilization (MOMP). Caspases are essential in cells for apoptosis, and have been termed “executioner” proteins for their roles in the cell. Caspases represent two central classes of molecules that are either involved with the stimulation of the apoptotic cascade (initiator caspases), or the various sequential biological pathways required for its execution (effector caspases). Some caspases are required in the immune system for the maturation of cytokines. However, latterly, evidence has accumulated that non-caspases, including calpains, cathepsins, granzymes and the proteasome have roles in mediating and promoting cell death.
In most cells, these pathways are controlled by the Bcl-2 family of proteins that can be divided into anti-apoptotic and pro-apoptotic members. In the mitochondrial pathway of apoptosis, caspase activation is closely linked to mitochondrial outer membrane permeabilization (MOMP). Caspases are essential in cells for apoptosis, and have been termed “executioner” proteins for their roles in the cell. Caspases represent two central classes of molecules that are either involved with the stimulation of the apoptotic cascade (initiator caspases), or the various sequential biological pathways required for its execution (effector caspases). Some caspases are required in the immune system for the maturation of cytokines. However, latterly, evidence has accumulated that non-caspases, including calpains, cathepsins, granzymes and the proteasome have roles in mediating and promoting cell death.
One unresolved problem in cell biology has been to explain how a cell dies rapidly while maintaining its energy levels, preserving the structure of its mitochondria and ER (endoplasmic reticulum), and not adversely affecting its neighbours. Failure of apoptosis is one of the main contributions to cancer development and autoimmune diseases. Numerous pro-apoptotic signal-transducing molecules and pathological stimuli converge on mitochondria to induce MOMP. The local regulation and execution of MOMP involve proteins from the Bcl-2 family, mitochondrial lipids, proteins that regulate bioenergetic metabolite flux, and putative components of the permeability transition pore. MOMP is lethal because it results in the release of caspase-activating molecules and caspase-independent death effectors, metabolic failure in the mitochondria, or both.
The pivotal step towards cell death is the formation of a pore in the mitochondria. In a healthy cell, the outer membranes of its mitochondria display the protein Bcl-2 on their surface. Bcl-2 inhibits apoptosis. Internal damage to the cell causes a related protein, Bax, to migrate to the surface of the mitochondrion where it inhibits the protective effect of Bcl-2 and inserts itself into the outer mitochondrial membrane punching holes in it and causing cytochrome c, which is the protein that initiates cell death, to leak out. Pore formation is the point of no return in apoptosis. Only two proteins are known to form the pore, Bak (BCL-2 antagonist or killer) and Bax (BCL-2-associated X protein). Bak and Bax promote apoptosis by perturbing the permeability of the mitochondrial outer membrane and facilitating the release of cytochrome c.
Mitochondria and cell death: outer membrane permeabilization and beyond.
Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway.
How do BCL-2 proteins induce mitochondrial outer membrane permeabilization?
The emerging role of serine proteases in apoptosis.
Guardians of cell death: the Bcl-2 family proteins.
Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis.
Proapoptotic BAX and BAK control multiple initiator caspases.
Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release.
In order to form the pore, Bak first changes shape and then combine with another Bak protein to form a doublet. Once the doublet is formed it can combine with other Bak doublets by what’s called a second interface. This second interface seems to allow doublets to assemble into the larger complexes that form the pore. Researchers to study how the large complexes of Bak and Bax force a hole in the mitochondrial membrane, how to start this process more effectively in cancer cells, and how to prevent it in brain and other healthy cells. A major black box in understanding apoptosis is how Bak and Bax work. Because these proteins change shape and lodge in a membrane they are hard to study. Any understanding we gain about how Bak and Bax form a pore, how they change shape and how they bind to each other, will help us understand how cancer cells can be targeted to die.
Induction of apoptosis is an important event for cancer treatment by naturally occurring dietary agents. Alpha-tocopheryl succinate (alpha-TOS) is the most effective form of vitamin E in comparison to alpha-tocopherol, alpha-tocopheryl acetate and alpha-tocopheryl nicotinate in inducing differentiation, inhibition of proliferation and apoptosis in cancer cells, depending upon its concentration. Alpha-TOS has already shown promise as a potent anticancer agent in diseases such as colon cancer, breast cancer (with high levels of HER2) and mesothelioma.
It induces controlled cell death or apoptosis in tumour cells. Study shows that alpha-TOS involves generation of reactive oxygen species (ROS). ROS then mediate the formation of disufide bridges between cytosolic Bax monomers, resulting in the formation of mitochondrial outer membrane channels. ROS also cause oxidation of cardiolipin, triggering the release of cytochrome c and its translocation via the activated Bax channels.
The following study shows that the combination of alpha-TEA, methylseleninic acid (MSA), and trans-resveratrol is more effective than single treatments for inhibiting cell proliferation, inducing cellular differentiation, and inducing cell death by apoptosis in cancer cells
 Dihydroartemisinin (DHA) is a semisynthetic derivative of the herbal antimalaria drug artemisinin. DHA is used to treat malaria. DHA is the active metabolite of all artemisinin compounds (artemisinin, artesunate, artemether, etc.) and is also available as a drug in itself. DHA is cytotoxic to tumor cells. Study shows that treatment of Jurkat T-lymphoma cells with DHA induced a breakdown of the mitochondrial transmembrane potential, release of cytochrome c, activation of caspases, and DNA fragmentation indicative of apoptosis induction. DHA treatment triggered the expression of NOXA and the activation of Bak. Furthermore, DHA-induced apoptosis was completely abrogated by loss of Bak and largely reduced in cells with siRNA-mediated downregulation of Bak or NOXA.
Dihydroartemisinin (DHA) is a semisynthetic derivative of the herbal antimalaria drug artemisinin. DHA is used to treat malaria. DHA is the active metabolite of all artemisinin compounds (artemisinin, artesunate, artemether, etc.) and is also available as a drug in itself. DHA is cytotoxic to tumor cells. Study shows that treatment of Jurkat T-lymphoma cells with DHA induced a breakdown of the mitochondrial transmembrane potential, release of cytochrome c, activation of caspases, and DNA fragmentation indicative of apoptosis induction. DHA treatment triggered the expression of NOXA and the activation of Bak. Furthermore, DHA-induced apoptosis was completely abrogated by loss of Bak and largely reduced in cells with siRNA-mediated downregulation of Bak or NOXA.
Dihydroartemisinin induces apoptosis by a Bak-dependent intrinsic pathway.