Cancer cells must satisfy the metabolic demands of fast-growing cancer within a continually changing microenvironment. The metabolism of cancer is approximately 8 times greater than that of normal cells and the body is constantly overworked trying to feed it. A fundamental difference in sugar metabolism exists between tumor cells found in the hypoxic regions of most solid tumors and the majority of normal cells in the body, which are under oxygen.
Sugar, or glucose, is brought into cells and converted into useable energy, a molecule called ATP. Another product of this conversion, a molecule called lactate, is then taken out of the cell by specialized transporters. Glucose is metabolized by glycolysis in a multi-step set of reactions resulting in the creation of pyruvate. In normal cells, much of this pyruvate enters the mitochondria where it is oxidized by the Krebs Cycle to generate ATP to meet the cell’s energy demands.
However, most cancer cells instead rely on aerobic glycolysis. Nobel winner Dr. Otto Warburg showed that cancer cells don’t rely heavily on the mitochondria to generate energy. Instead, they obtain as much as 50% of their ATP by metabolizing glucose directly to lactic acid, even when oxygen is available. This phenomenon of cancer became known as the “Warburg effect.” This metabolic adaptation supports rapid cell proliferation.
Cancer cells frequently use glutamine as a secondary fuel source, which enters the mitochondria and can be used to replenish Krebs Cycle intermediates or can be used to produce more pyruvate through the action of malic enzyme. This process is called glutaminolysis.
The oncogenic transcription factor c-MYC stimulates glutamine catabolism to fuel growth and proliferation of cancer cells through up-regulating glutaminase. In general, c-MYC alone does not cause cancer. But it does stimulate the hyperproliferation of various cells. Hyperactivated c-MYC has been identified in 80% of all cancers and leukemias. Glutamine is converted to glutamate by glutaminase and then metabolized to α-ketoglutarate, a key metabolite of the TCA cycle. Less well-recognized, glutamate can also be converted to proline.
This metabolic change is associated with the over-expression of all the pathway enzymes and transporters (as induced by HIF-1α and other oncogenes), and with the expression of hexokinase (HK) and phosphofructokinase type 1 (PFK1) enzymes with different regulatory properties. The hypoxia-inducible factor (HIF-1), in addition to genetic and epigenetic changes, is largely responsible for alterations in cell metabolism in hypoxic tumor cells. This creates a natural window of selectivity that can be exploited for therapy by using inhibitors of glycolysis.
Cancer as a metabolic disease.
Understanding the Warburg effect: the metabolic requirements of cell proliferation.
Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity.
The proline regulatory axis and cancer.
Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells.
Hypoxia and energetic tumour metabolism.
Highly proliferative cancer cells need to produce excess nucleotide, lipid, and amino acids for the creation of new biomass. Glycosylation (not to be confused with glycolysis) is the adding of a sugar (polysaccharide or oligosaccharide) to proteins, lipids, or other organic molecules. Cancer is associated with glycosylation alterations in glycoproteins and glycolipids.
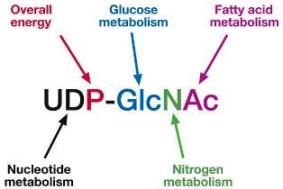 A new research has found that tumor cells alter glycosylation, the addition of carbohydrates (in this case GlcNAc) to their proteins, in response to their surroundings. This ultimately helps the cancerous cells survive. When the scientists blocked the addition of GlcNAc, a specific sugar that is pronounced “glick-nack”, to a particular protein in mice, tumor-cell growth was impaired.
A new research has found that tumor cells alter glycosylation, the addition of carbohydrates (in this case GlcNAc) to their proteins, in response to their surroundings. This ultimately helps the cancerous cells survive. When the scientists blocked the addition of GlcNAc, a specific sugar that is pronounced “glick-nack”, to a particular protein in mice, tumor-cell growth was impaired.
Researchers have found that when GlcNAc attaches to PFK1 (phosphofructokinase type 1), it suppresses glycolysis at an early phase and reroutes the products of previous steps into a different pathway—one that yields the nucleotides a tumor needs to grow, as well as molecules that protect tumor cells. So GlcNAc causes tumor cells to make a trade—they produce fewer high-energy compounds in order to get the products they need to grow and survive. What’s unique here is that the addition of GlcNAc is dynamic and reversible. This allows a cancer cell to more rapidly alter its metabolism depending on the environment that it encounters.
Researchers have found that this glycosylation—the addition of GlcNAc to PFK1—is enhanced under conditions associated with tumors, such as low oxygen levels. They also found that glycosylation of PFK1 was sensitive to the availability of nutrients. If certain nutrients were absent, glycosylation was increased, and the tumor was able to compensate for the dearth of nutrients by changing the cell’s metabolism.
When the researchers analyzed human breast and lung tumor tissues, they found GlcNAc-related glycosylation was elevated two- to fourfold in the majority of tumors relative to normal tissue from the same patients. Then, working with mice injected with human lung-cancer cells, the researchers replaced the existing PFK1 enzymes with either the normal PFK1 enzyme or a mutant form that could no longer be glycosylated. The mice with the mutant form of PFK1 showed decreased tumor growth, demonstrating that blocking glycosylation impairs cancerous growth.
The work suggests at least two possible avenues for future investigations into fighting cancer. One would be to develop compounds that prevent PFK1 from becoming glycosylated, similar to the mutant PFK1 enzymes in the present study. The other would be to activate PFK1 enzymes in order to keep glycolysis operating normally and help prevent cancer cells from altering their cellular metabolism in favor of cancerous growth.
Altered glycosylation of proteins in cancer: what is the potential for new anti-tumour strategies.
Phosphofructokinase 1 glycosylation regulates cell growth and metabolism.
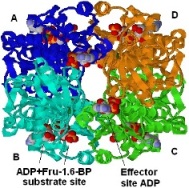 As discussed above, PFK1 plays a pivotal role in the regulation of glycolysis through the Embden-Meyerhof pathway. The first reaction that commits glucose to the glycolytic pathway is catalyzed by the enzyme PFK1. The activity of PFK1 is known to be regulated by the AMP (adenosine 5’-monophosphate) to ATP (adenosine 5’-triphosphate) ratio and citrate, but, in addition, it also is known to be regulated by the intracellular level of F2,6BP (fructose 2,6-bisphosphate), which is a product of another enzyme, PFK2/FBP2 (6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase). Among other effectors, citrate has been reported to play a vital role in the suppression of PFK1 activity. PFK1 activity has important consequences for several different aspects of cell metabolism as well as for systemic metabolic conditions. PFK1 can bind several allosteric effectors.
As discussed above, PFK1 plays a pivotal role in the regulation of glycolysis through the Embden-Meyerhof pathway. The first reaction that commits glucose to the glycolytic pathway is catalyzed by the enzyme PFK1. The activity of PFK1 is known to be regulated by the AMP (adenosine 5’-monophosphate) to ATP (adenosine 5’-triphosphate) ratio and citrate, but, in addition, it also is known to be regulated by the intracellular level of F2,6BP (fructose 2,6-bisphosphate), which is a product of another enzyme, PFK2/FBP2 (6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase). Among other effectors, citrate has been reported to play a vital role in the suppression of PFK1 activity. PFK1 activity has important consequences for several different aspects of cell metabolism as well as for systemic metabolic conditions. PFK1 can bind several allosteric effectors.
Control of glycolysis through regulation of PFK1: old friends and recent additions.
Evolution of allosteric citrate binding sites on 6-phosphofructo-1-kinase.
Understanding the central role of citrate in the metabolism of cancer cells.
Herbalzym Vinegar is made from a powerful blend of dried persimmon, wild medicinal mushrooms, raw black honey, and other all-natural herbs and extracts, including GenisZym and Rhubarb extracts. The objective of Herbalzym Vinegar Therapy (HVT) is to alter or impair the development of cancer cells by interfering with cancer cell metabolism.
 Herbalzym Vinegar is rich sources of several compounds that help shut down glycolysis and cut off the energy supply to cancer cells. Keep in mind, it is much more than just a “diet”; it is a glucose reduction protocol to impair the development of cancer cells, thus starving them to death. Clinical trials have already been done with humans using Herbalzym Vinegar (metabolic modulation formulas), and with much success.
Herbalzym Vinegar is rich sources of several compounds that help shut down glycolysis and cut off the energy supply to cancer cells. Keep in mind, it is much more than just a “diet”; it is a glucose reduction protocol to impair the development of cancer cells, thus starving them to death. Clinical trials have already been done with humans using Herbalzym Vinegar (metabolic modulation formulas), and with much success.
Herbalzym Vinegar has been proven its power to modulate PFK1 activity which shuts down the energy supply to cancer cells, simultaneously enhancing the benefits of chemotherapy while lessening their toxic side effects. It can also increase p53 activity, considered the single most important tumor-suppressor gene, while inhibiting c-MYC expression. What more can you ask for?
Herbalzym Vinegar is a natural product that increases the production of both acetic acid and citric acid in the body. Acetic acid and citric acid also help fight cancer by shutting down the process of glycolysis. Normal cells derive most of their daily energy supply from acetic acid and citric acid, where as cancer cells derive most of their daily energy from glycolysis. Herbalzym Vinegar is an excellent anti-cancer and anti-leukemia natural medicine.